Research
Radiation chemistry impacting nuclear power generation
-
Kinetics and mechanisms of radical reactions in aqueous solutions at various temperatures – nuclear reactor water radiolysis
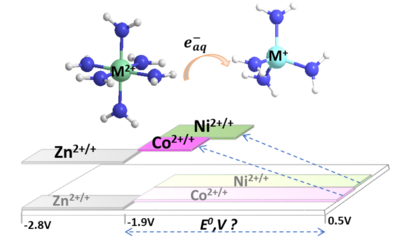
We explore kinetics and mechanisms of radical reactions at high temperatures – reactor water radiolysis and pulse radiolysis studies.It is well nown that coolant water in a nuclear reactor receives high doses of radiation, gamma rays, and fast neutrons. This project is devoted to complex research related to radiation chemistry of nuclear reactor water and ions dissolver in water under high temperatures and pressures. The obtained data will be used for the modeling of the water chemistry under typical pressurized and boiling water reactors conditions in order to improve the safety and reliability of nuclear power generation. Reactions of Nickel Ions in Water Radiolysis up to 300 °C Hig-Temperature Reaction Kinetics of the eaq– and HO2• Radicals with Iron(II) Ions in Aqueous SolutionsKinetics of the Temperature‐Dependent eaq− and ⋅OH Radical Reactions with Cr(III) Ions in Aqueous Solutions Reactivity of Zn+ aq in high-temperature water radiolysis
Pulse Radiolysis and Transient Absorption of Aqueous Cr (VI) Solutions up to 325° COne-electron redox kinetics of aqueous transition metal couples Zn2+/+, Co2+/+, and Ni2+/+
Reduction of CO2 by hydrated electrons in high temperature water
Partial molar volume of the hydrated electron
-
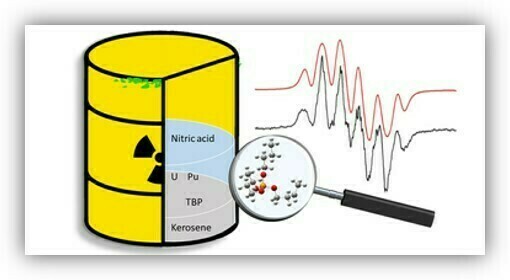
The radiolytic stability of the separation systems in the nuclear waste reprocessing
We apply EPR spectroscopic techniques to characterize radicals derived from compounds that have applications ranging from those relevant to the nuclear power industry to photochemistry of importance in biology and medicine. In our research, we evaluate the radiation stability of ionic liquids and organic ligands used in the separation systems in the nuclear waste reprocessing. We find the structures of radicals formed upon irradiation of ionic liquids and /or ligands, which may call into question their use for the extraction of spent nuclear fuel.
Assessing the radiation stability of key ligands in nuclear waste separationRadicals from tributyl phosphate decomposition: a combined electron paramagnetic resonance spectroscopic and computational chemistry investigationPersistent radicals in irradiated imidazolium ionic liquids probed by EPR spectroscopyγ-Radiolysis of Room-Temperature Ionic Liquids: An EPR Spin-Trapping Study
Free radical chemistry & Redox chemistry of bioorganic molecules
We have developed collaborations across national and international laboratories to broaden the horizons of our research and bring additional expertise.
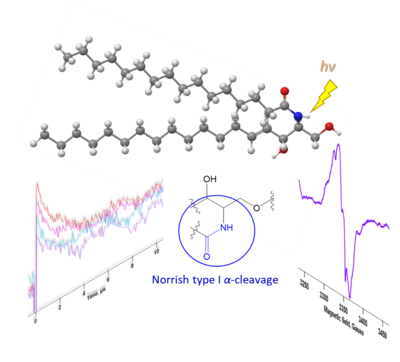
One idea involves attempts to develop a detailed understanding of free radical mechanisms of lipid degradation, which are closely associated with various physiological and pathological processes. The idea is to identify the radicals generated by the action of reactive radicals on lipids (UV light and hydroxyl radical derivatives). Unveiling the Mechanism of Photodamage to Sphingolipid Molecules via Laser Flash Photolysis and EPROH radical reactions with the hydrophilic component of sphingolipids ROS-induced lipid transformations without oxygen participation